Analysis of Aspirin Tablets Experiment
The aim of this experiment is to ascertain the number of moles of aspirin acetylsalicylic acid which is manifest in five distinct tablets providing titration for the compound with a sodium hydroxide solution 0100 dm-3 as a base. The binder is chemically inert and was intentionally added by the.
Analysis Of Aspirin Tablets On A Microscale Experiment Rsc Education
CHM250 Analysis of Aspirin Page 1 Introduction.

. Other solution concentration units that will be used in this experiment. Aspirin is the trade name for acetylsalicylic acid ASA. Experiment 6 Analysis of Aspirin Tablets Introduction Pharmaceutical manufacturers are required by law to state on the packaging the amount of each active ingredient in their products.
6 ANALYSIS OF THE ASPIRIN TABLET Unknown a. This can be done by dissolving a tablet in a strong base NaOH and titration it with a strong acid HCl. ANALYSIS OF ASPIRIN TABLETS INTRODUCTION Pharmaceutical.
In this experiment we will determine the percent active compound in a commercial aspirin tablet. Students use a UV-Vis spectrometer to determine the mass of acetylsalicylic acid ASA in a single aspirin tablet and compare the results to the ASA content stated on the aspirin bottle. Rinse the 2 ml GRADUATED pipet with 1-2 ml of the unknown tablet solution.
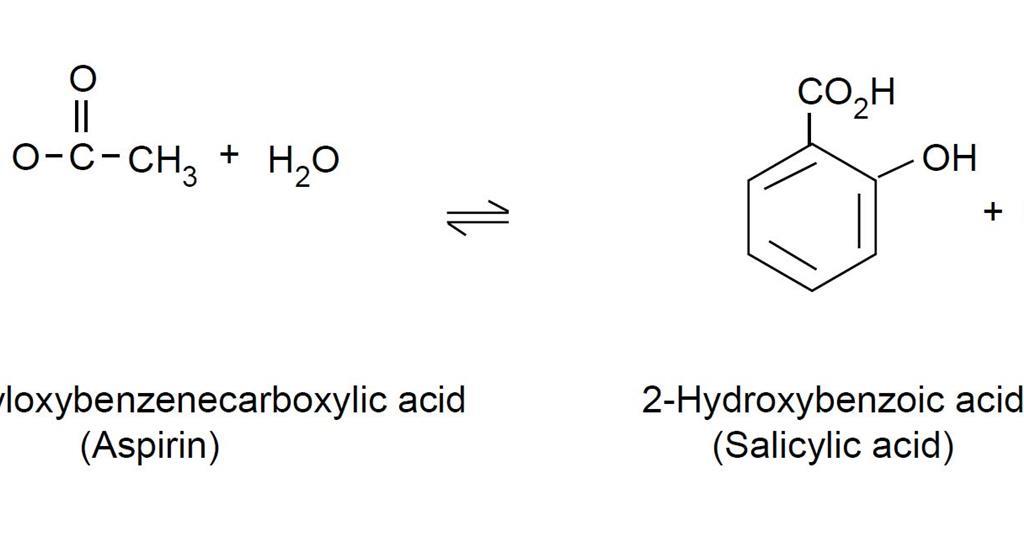
ANALYSIS OF ASPIRIN TABLETS The aim of this investigation is to determine the percentage by mass of aspirin aspirin present in different commercial preparations and to find out which the best value is using a neutralization reaction followed by a direct titration with NaOH. 100 ml of distilled water in a wash bottle 50 ml of 0. In todays experiment we will measure the intensity of the colored complex that forms when iron III is mixed with aspirin to determine the amount of pure aspirin acetylsalicylic acid in commercial aspirin tablets.
The spectroscopic analysis of aspirin will involve the complexing of ironIII to the deprotonated form of salicylic acid salicylate ion to give a purple solution. In Part I of this experiment you will prepare aspirin by reaction of salicylic acid. Analysis of Aspirin Tablets.
In this experiment a consumer survey on. It is a drug that was first isolated in Germany by the Bayer Company in 1897. It has the ability to reduce fever an antipyretic to.
Experiment 11 Synthesis and Analysis of Aspirin INTRODUCTION Aspirin is most widely sold over-the-counter drug. Determination of Aspirin using Back Titration This experiment is designed to illustrate techniques used in a typical indirect or back titration. Your aspirin product as well as a commercial aspirin tablet will be compared to a standard 015 ferric-salicylate solution.
The determination will be conducted if the acid possesses a robust of feeble aspect in accordance with the Lewis. The content of active ingredient in a tablet will always be stated on the package. Obtain your unknown solution from your TA and record the unknown number.
Only the salicylate ion complexes to ironIII. _ EXPERIMENT 7. More than 50 million 5-grain tablets of aspirin are consumed daily in the United States.
Learn how a UV-Vis Spectrometer can be used to analyze the amount of acetylsalicylic acid ASA in an aspirin tabletGet the free student and teacher experim. Experiment the total number of. Most aspirin tablets contain a small amount of binder which helps prevent the tablets from crumbling.
EXPERIMENT 2- ANALYSIS OF ASPIRIN MATTHEW SKILTON DUE MONDAY FEBRUARY 13TH 2012 TA- ANA GARGAUN ABSTRACT In this experiment the concentration of acetyl salicylic acid ASA in an aspirin tablet will be determined. To calculate and compare the active pharmaceutical ingredient API in different commercially available aspirin tablets of the same batch using titration technique. The ASA in the tablet will be reacted with Fe3 forming an intensely violet coloured complex.
The stockroom will make the UNKNOWN by dilut ing ONE COMERCIAL ASPIRIN TABLET to 100 mL solution. Spectrophotometric analysis of a commercial Aspirin tablet. View Lab Report - ANALYSIS OF ASPIRIN TABLETS from CHEM 213 at University of the Fraser Valley.
Experimental general chemistry 103. The chemical name for aspirin is Acetyl salicylic acid ASA. The quantity was found to.
You will be graded on your accuracy. The Beer-Lambert Law often shortened to Beers Law relates the absorbance of a sample to the concentration of. Commercially prepared aspirin tablets are not considered 100 pure acetylsalicylic acid.
Determination of aspirin in tablets using back titration Aim. You will use the NaOH you standardized last week to back titrate an aspirin solution and determine the concentration of aspirin in a typical analgesic tablet. It is used as a fever reducer pain reliever and has antiplatelet effects.
The amount of the active ingredient 2 ethanoyloxybenzoic acid or o acetylsalicylic acid in different commercial brands of aspirin. Identify questions and make predictions that can be addressed by conducting.

Aspirin Tablets Titration Titration Of Aspirin Tablets In This Lab You Will Determine The Percent Studocu


No comments for "Analysis of Aspirin Tablets Experiment"
Post a Comment